Remote Monitoring
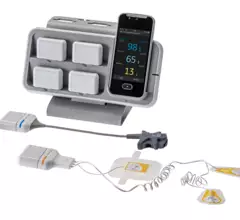
Remote cardiac monitoring technologies enable patient health to be tracked outside the clinical setting. It can be used for longer term monitoring to help diagnosis arrhythmias or other cardiac conditions. Remote monitoring also can keep tabs on chronic conditions such as heart failure or hypertension and alert clinicians to worsening symptoms to avoid an acute care episode or hospitalization.
Displaying 9 - 16 of 21