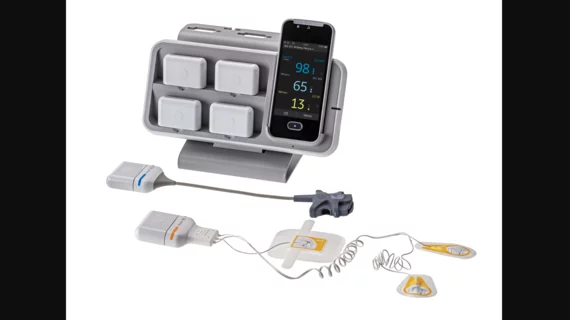
GE HealthCare earns FDA clearance for new remote patient monitoring device
GE HealthCare has received clearance from the U.S. Food and Drug Administration (FDA) for its Portrait Mobile wireless patient monitoring solution, which allows for continuous monitoring of vital signs regardless of a patient’s location. The company says it hopes clearance from the FDA will carve a path to wider adoption that could lead to better care delivery outcomes.
The Portrait Mobile system is a wearable sensor for remotely tracking vital signs. A notable feature of Portrait Mobile is its capability to consistently capture respiration rate, an indicator often sensitive to early signs of patient deterioration. The sensors employ a dual-vector measurement approach, aimed at accurate tracking of a patient’s respiration rate, even as breathing patterns fluctuate.
“Patients recovering from major surgery are fragile. Most patients currently have vital signs monitored every four-six hours. We have shown that vital sign abnormalities are common – and sometimes profound and prolonged,” Daniel Sessler, MD, Michael Cudahy professor and chair of outcomes research with Cleveland Clinic, said in a statement. “Many potentially serious episodes of instability are missed with intermittent vital sign assessments.”
Sessler is overseeing a trial evaluating Portrait Mobile. He emphasized the potential of the technology to facilitate early interventions for patients at risk: “Continuous vital sign monitoring might help clinicians identify patients who are having difficulty so they can provide help quickly.”
Undetected patient deterioration, especially after surgery, is associated with preventable complications, according to GE Healthcare. Continuous monitoring of vital signs such as respiration rate, oxygen saturation, and pulse rate provides healthcare teams with the ability to respond promptly to changes in a patient's health.
“It’s important for recovery that patients be able to move around freely while their vital signs are being monitored,” Neal Sandy, general manager of monitoring solutions for GE HealthCare, said in the same statement. “Until Portrait Mobile, patient monitoring required that patients be tethered to their beds, limiting mobility. GE HealthCare designed Portrait Mobile with this need in mind – the advent of a small wearable, wireless inpatient monitoring solution that provides reliable monitoring to the patient's care team, while allowing for more patient freedom and flexibility during recovery, is an important advancement in acute care.”
The design and approach of Portrait Mobile have received recognition, including the iF Design Gold Award for Product Design in 2022.