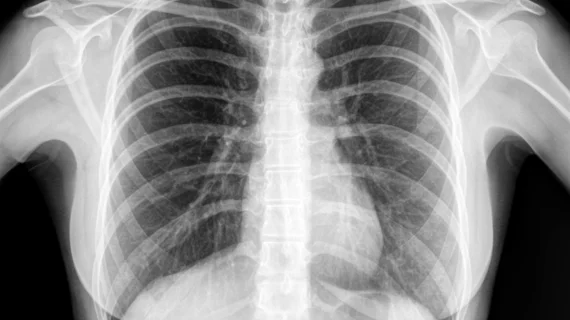
Google canceled release of 100K images after NIH intervention
Just two days before Google was set to release an expansive set of human chest X-ray images, the National Institutes of Health (NIH) called the tech giant to call it off over privacy concerns, the Washington Post reported. Personal information about the patients in the images were still contained in some of the files and could be used to identify patients, NIH told Google.
The images were meant to be publicly posted in 2017, and the incident was discovered through a Freedom of Information Act request from the Washington Post. Google then canceled its project with NIH, and Google did not obtain legal agreements “covering the privacy of patient information, during the planning of the X-ray project with NIH.
Google’s intent was to showcase its cloud computing services by publicly hosting the images, which included 112,000 chest X-ray images from more than 30,000 patients, many with lung disease, the Washington Post found in its records request.
The revelation underscores the rising privacy and legal concerns about Google’s ever-increasing forays into the healthcare space. Another major health project involved the health data of millions of U.S. patients was revealed mid-November this year, this time between Google and Ascension. Google has stated both projects are in compliance with federal privacy laws and no rules were broken.
See the full story below: