FDA issues warning on endoscopes after injuries, death from infections
The U.S. Food and Drug Administration (FDA) has issued an alert due to a high-risk issue with certain endoscopes manufactured by Olympus. The FDA’s notice follows a letter the company sent to customers on December 18 after reports of 120 injuries and 1 death related to infections caused by the endoscopes.
While no formal recall has been issued, these communications could be a precursor. The FDA said its late-December alert is part of the process for its Communications Pilot to Enhance the Medical Device Recall Program.
The issue appears to affect all lots of MAJ-891 endoscopes, with infections linked to an issue with the forceps and irrigation plug, which are difficult to clean and sanitize. Reprocessing these particular endoscopes is common, as they are reusable. If not disassembled before sterilization, microbes can remain alive, leading to illnesses such as sepsis and urinary tract infections, which have been serious enough to cause hospitalizations.
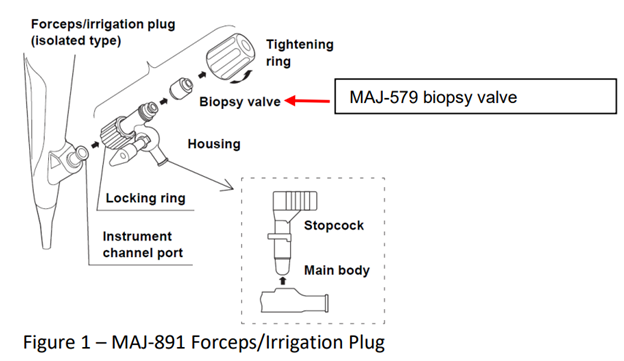
Olympus has advised providers to seek alternative endoscopes, due to the issues with properly reprocessing the MAJ-891. However, it added there are no alternative irrigation plugs for the scopes, meaning providers may have to replace the scopes outright.
To avoid a replacement, Olympus is asking providers to ensure the scopes are completely disassembled before being cleaned and sanitized, in accordance with the device’s instructions. They’re also recommending the biopsy valve be inspected before each use to ensure there is no damage or corrosion.
The affected scopes all share a unique device identifier (UDI): 04953170063114.
Any questions or reports of adverse reactions should be addressed to Olympus by calling its Technical Assistance Center at 800-848-9024, option 1.
The full recall notice—including the full list of scopes covered under this alert—can be found by clicking here.

