FDA announces recall of endoscope sheaths after 26 injuries
The U.S. Food and Drug Administration (FDA) has announced an urgent recall on behalf of Olympus for certain models of its single-use guide sheath kits. The alert comes after reports of the radiopaque tip of the devices falling off into patients.
These instruments are designed for use with an endoscope to collect cells or tissue from organs for testing, in this case, from parts of the body responsible for breathing.
After an investigation, the company determined the tip was detaching when force was applied as instruments were inserted into the sheaths. Additionally, potential damage to the distal end of the sheaths could cause the tip to disconnect.
To date, there have been 26 serious injuries associated with their usage, but no deaths. Due to a risk of serious, potentially fatal bleeding, the recall has been designated as Class I, the FDA’s most serious type.
What is being recalled?
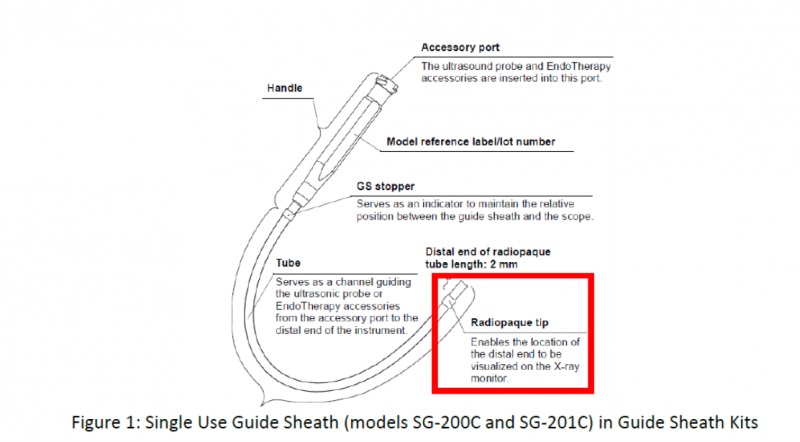
The guide sheaths from Olympus are sold in kits, with models K-201, K-202, K-203 and K-204 containing the SG-200C and SG-201C single-use sheaths, which are the target of the recall. Below are the specific products impacted:
- K-201 2.0MM Channel Set: Guide Sheath, Biopsy Forceps, Cytology Brush
- K-202 2.0MM Channel Set: Guide Sheath, Biopsy Forceps
- K-203 2.6MM Channel Set: Guide Sheath, Biopsy Forceps, Cytology Brush
- K-204 2.6MM Channel Set: Guide Sheath, Biopsy Forceps
Unique Device Identifier (UDI) and model numbers:
- 04953170245466/K-201
- 04953170245480/K-202
- 04953170245503/K-203
- 04953170245527/K-204
Next Steps
Use of the recalled kits must cease immediately, given the high risk to patient safety. Olympus notified all known customers of the recall on January 15 in an Urgent Medical Device Removal letter. In the letter, they asked providers to identify and quarantine single-use sheaths in the recalled kits.
Customers are asked to call 1-800-848-9024, option 2, to initiate a return. Olympus stated it will issue a credit upon return of the affected product.
Both the company and the FDA are asking those who received the letter to share and forward it to other users who may be impacted by the recall.
The notice from the FDA can be found here.

