FDA issues Class I recall on insulin syringes due to risk of death
The U.S. Food and Drug Administration (FDA) is spreading the word about recalled lots of insulin syringes manufactured by Cardinal Health. A line of Monoject U-100 1 mL insulin syringes has an apparent manufacturing defect that leaves them incompatible with needleless IV connectors. If used to administer insulin, there is a risk the patient will not receive the full dose, as fluids may fail to leave the syringe.
Due to the risk posed by patients receiving an inadequate dose of insulin—including hypoglycemia and death—the FDA has given this recall a Class I designation, reserved for the most serious cases. There have been no reported injuries or deaths.
Cardinal Health alerted known customers directly about the recall in a letter sent on Sept. 25. However it is possible not all of the syringes were removed from supplies and clinical settings. Details on the recall are below.
What is being recalled?
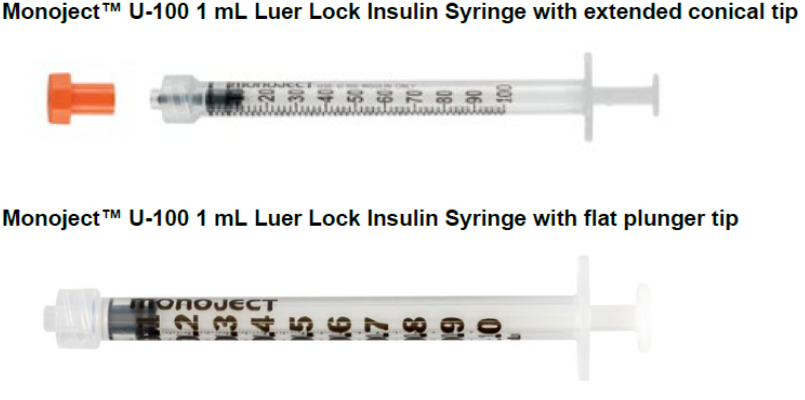
The product in questions is Monoject U-100 mL Insulin Syringe Luer Lock with Tip Cap Soft Pack—specifically those with the extended conical tip. The associated code is 1199100777 and the following lots are affected:
Lot Numbers:
- 221201
- 230201
- 230202
Unique Device Identifier (UDI):
- (01)10192253034783 (EA)
- (01)20192253034780 (BX)
- (01)50192253034781 (CS)
If any patient or clinician has a syringe with any of these lot or UDI numbers, they must not be used to push insulin through any IV connector.
Next Steps
Cardinal Health is asking for an inventory check from anyone who may be in possession of the recalled syringes. They should not be used. Instead, seek a replacement by contacting the company at GMB-FieldCorrectiveAction@cardinalhealth.com
The FDA and Cardinal Health ask that this alert be shared to spread awareness. The full recall notice can be found here.

