FDA announces emergency recall of oxygen concentrators after spontaneous fires
The U.S. Food and Drug Administration (FDA) has issued a Class I recall—the most serious type—on a line of oxygen concentrators manufactured by Jiangsu Jumao X-Care Medical Equipment after incidents of the devices catching fire and melting during use.
While there have been no reported injuries or deaths, the devices are to be removed from the market, with use by patients and service to the units immediately discontinued.
The oxygen concentrators, used to provide support to patients with respiratory disorders, work by separating nitrogen from room air by way of a molecular sieve. Patients who require them for supplementary oxygen will need to return the unit and seek an immediate replacement.
The cause of the spontaneous combustion and resulting fires is unknown and currently being investigated by the FDA and Jiangsu Jumao.
What is being recalled?
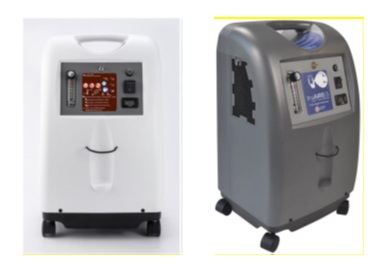
- Product Names: Trueaire-5 02 Concentrator
- Model: O2C5L
- Serial Numbers: All units within serial number range: JA2311000001-JA2312000740
Next Steps
All units from the specified serial number range must be immediately removed from service. They should then be promptly returned to Compass Health Brands, the parent company of the manufacturer.
Jiangsu Jumao sent all known affected customers an Urgent Medical Device Recall letter on November 26, 2024. In it, they ask that anyone in possession of an oxygen compressor subject to the recall fill out and submit a response form, even if they have no receipt or record of purchasing the unit.
Customers in the U.S. with questions are asked to contact Compass Health at 1-800-376-7263, extension 444.
Both the company and the FDA are also asking that the recall notice be shared. It can be viewed here.

