FDA recalls infant incubators due to risk of door falling off
A Class I recall has been issued by the U.S. Food and Drug Administration (FDA) on behalf of GE HealthCare for a faulty door on a line of infant incubators. Due to a risk of injury to newborns, the recall falls into the most serious category. However, there have been no reported injuries or deaths.
A faulty screw on Giraffe OmniBed and Giraffe OmniBed CareStation—both produced by GE subsidiary Datex-Ohmeda—is to blame, and the company is asking providers, clinicians and caretakers to monitor and check the heater doors before use to ensure their safety. There is a risk the door could come loose, setting off an alarm and potentially even falling onto an infant patient.
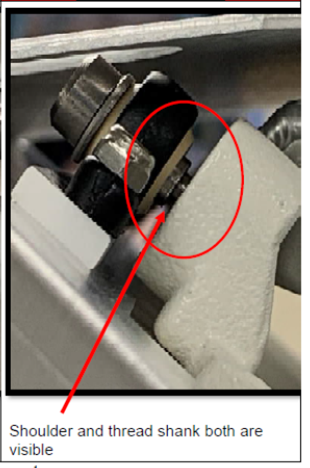
To conduct a safety check, simply look at the heater door hinges to ensure the screw threads are not visible. See the below photo for details.
What is being recalled?
All models of the Giraffe OmniBed and Giraffe OmniBed CareStation will require the additional safety inspection before use.
See a full list of serial numbers subject to the recall here.
Next steps
There is no need to remove the beds from care settings, as they are otherwise safe if the screw on the heater door is tightly secured. If an alarm goes off or the screw thread is visible, the door could pose a danger to infants. Do not use the bed and seek repairs immediately.
Any customer with a recalled bed is asked to contact GE HealthCare at 1-800-437-1171 to ask questions or request a repair.
The full recall notice and updated use instructions can be found here.

