FDA announces Class I recall for compounding inlets due to risk of death
The U.S. Food and Drug Administration (FDA) has issued a Class I recall on behalf of Baxter Healthcare for the company’s disposable inlets, used with ExactaMix automated compounding systems. The recall follows reports of unwanted particulate matter found within the inlets, including the sterile tubing.
In response, the FDA and Baxter are issuing instructions on how to inspect and safely use the recalled components. The disposable inlets are for compounding drugs in hospital or clinic pharmacies before they are administered intravenously to patients. To date, there have been no reported injuries as a result of the buildup of particulate matter; however, due to the risk of serious adverse health consequences—including the potential to kill a patient—the recall is ranked as “most serious.”
What is being recalled?
The following lots of Baxter disposable inlets are subject to the recall. The ExactaMix automated compounding systems themselves are perfectly safe to use, with the issue surrounding unwanted particulate matter stemming from the inlets and associated components.
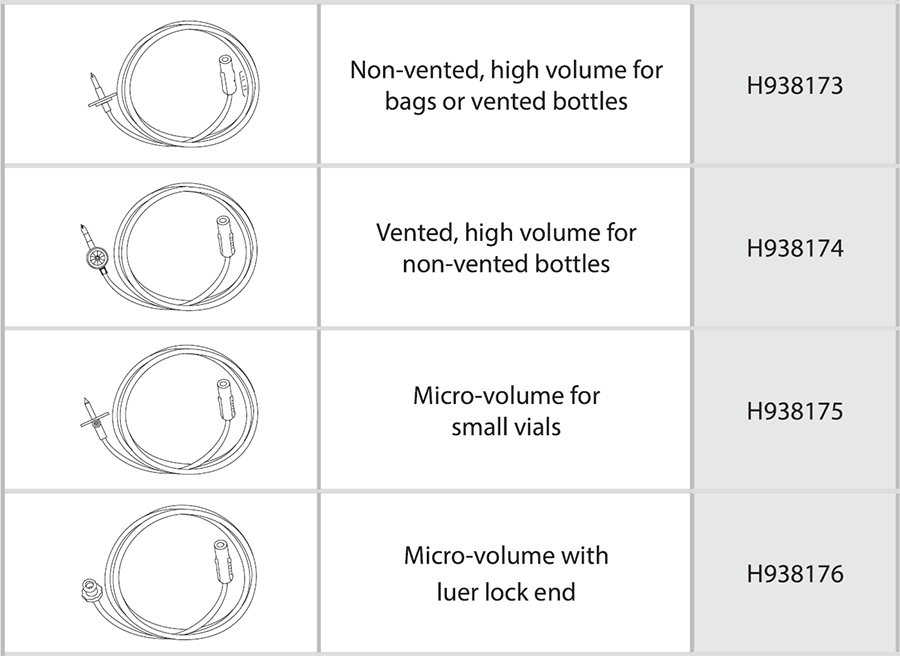
- Product Name: Automated Compounding Device Inlets (disposable inlet), used with the ExactaMix and ExactaMix Pro
- Product Codes: H938173, H938174, H938175 and H938176
- UDI: 00085412475783, 00085412475790, 00085412475806 and 00085412475813
For more details and a full list of affected components, click here.
Next steps
There is no need to discard the recalled inlets. However, all of the inlet—including the primary packaging, tubing, connectors and spikes—must be inspected for particulates before use. If any abnormality is observed, discard them and contact Baxter for a replacement using their web portal.
If the disposable inlets are inspected and do not contain any foreign material or particulate buildup, they can be used as normal.
To help propagate these instructions, the FDA requests the recall notice be posted so pharmacists and clinical staff are made aware of the issue. The recall notice can be found here.
Baxter also sent out a letter on Aug. 20 alerting known customers to the issue.

