3D-printed models for heart valve disease predict leaks in TAVR
3D printing and computer modeling could predict paravalvular leak (PVL) in patients undergoing transcatheter aortic valve replacement (TAVR), according to a study presented at the Society for Cardiovascular Angiography and Interventions (SCAI) Scientific Sessions.
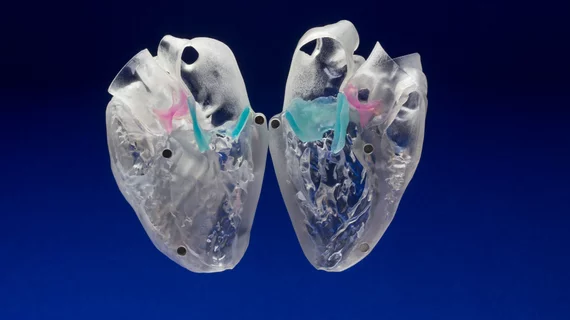
Individuals diagnosed with high-risk, inoperable narrowing of the aortic valve often undergo TAVR to replace the heart’s aortic valve. However, these patients are prone to paravalvular leaks that can lead to increased mortality rates. In this study, researchers evaluated the feasibility and impact of using 3D-printing technology to recreate patients’ hearts and predict where a potential leak could occur.
Six patients undergoing TAVR for calcific aortic stenosis at risk for a paravalvular leak were included in the study where they underwent computed tomography (CT) images to produce segments of their 3D-modeled heart at the location of the calcium buildup. The 3D aortic root models were then implanted in the valve print to determine if the sizing was off and compared to in-vivo implanted TAVR echocardiograms.
The 3D models showed the exact same leaks present in the CT digital scans. The accuracy of the models allowed researchers to create more personalized models for size, placement and location to prevent additional leaks and reduce calcium buildup.
"We are very encouraged to see such positive outcomes for the feasibility of 3D printing in patients with heart valve disease. These patients are at a high risk of developing a leak after TAVR, and anything we can do to identify and prevent these leaks from happening is certainly helpful," said lead author Sergey Gurevich, MD, and Cardiovascular Fellow at the University of Minnesota in Minneapolis, Minnesota. "Like any other new technology, as 3D printing evolves, we hope to see an increase in accessibility and opportunity for the use of this technology to help improve patient care."