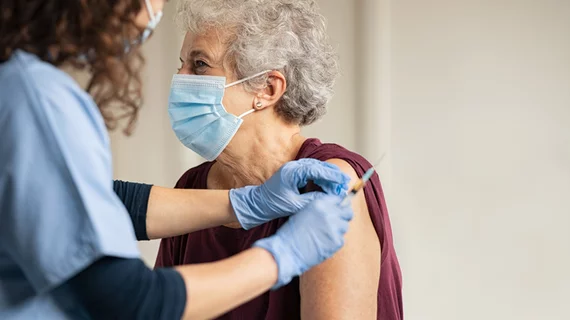
COVID-19 vaccines prevented 300K deaths in 2021
COVID-19 vaccines were effective at preventing serious illness and excess deaths in 2021, according to new data from the Department of Health and Human Services (HHS).
In fact, vaccines likely prevented 300,000 deaths and 650,000 hospitalizations among Medicare beneficiaries, HHS found. More than 90% of seniors are vaccinated against COVID-19, underscoring the vaccination program success and efficacy of the vaccines. HHS conducted a study with its Office of the Assistant Secretary for Planning and Evaluation (ASPE) to determine how many lives were saved from vaccines among the Medicare population.
"This report reaffirms what we have said all along: COVID-19 vaccines save lives and prevent hospitalizations," HHS Secretary Xavier Becerra said in a statement. "We now have updated COVID vaccines designed to protect you against the Omicron strain of COVID that makes up almost all COVID cases in the U.S. The Biden-Harris Administration has ensured that updated vaccines are available at tens of thousands of locations nationwide.”
The findings come as the Biden administration is encouraging folks to get a boost vaccine dose this fall. The U.S. Food and Drug Administration (FDA) recently approved a new booster that is effective in preventing serious disease from two recent COVID-19 subvariants.
“Over 90[%] of Americans live within 5 miles of where they can access these vaccines for free,” Becerra said. “I urge everyone eligible to get an updated COVID vaccine to protect yourself ahead of the fall and winter."
In addition to preventing thousands of deaths, the COVID-19 vaccines have saved $16 billion in direct medical costs as a result of reductions in hospitalizations, HHS noted. The savings and health impacts were seen across demographics and all 50 states.