FDA recalls Medtronic video laryngoscopes due to risk of explosion
The U.S. Food and Drug Administration (FDA) has issued a recall on behalf of Medtronic for several of the company’s video laryngoscopes due to multiple errors that could result in injury to a patient or clinician, and possibly even death.
Video laryngoscopes are used during a variety of medical procedures to allow clinicians to view a patient’s trachea. They include a light and a small camera. Due to the high risk a malfunction poses to patients, the recall of these Medtronic video laryngoscopes has been ranked as the "most serious." To date, one person has been reportedly injured.
The recall is somewhat complicated, as it requires certain McGrath MAC Video Laryngoscopes be removed from the market completely. Others simply require device users to follow updated instructions. In any case, a risk of battery depletion leaving the device not working—in addition to a risk of battery explosion—means anyone using Medtronic video laryngoscopes will want to take notice of this recall.
What is being recalled?
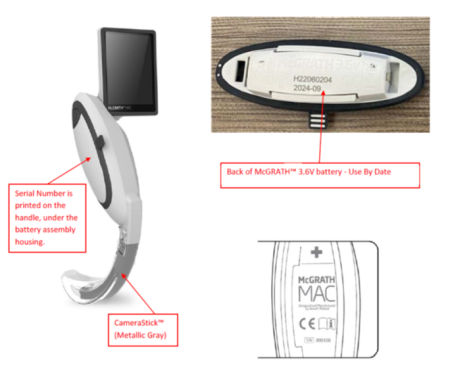
The FDA and Medtronic are instructing operators to stop using these devices immediately, and to remove the battery unit and discard it. These laryngoscopes will need to be returned to Medtronic for a replacement.
- Product Names: McGrath MAC Video Laryngoscope, McGrath MAC EMS Video Laryngoscope
- Unique Device Identifier (UDI)/Model: 15060272980020/300-000-000 and 15060272980129/300-200-000
- Serial Numbers: All serial numbers between 366170 to 405673
If you’re using one of the below video laryngoscopes, there is no need to discard anything and they can still be used. Instead, ensure the battery has been properly stored, per usage instructions and that the battery is still dated for safe use. If not, the battery should be discarded and replaced with a new one immediately.
- Product Name: Next-Generation McGrath MAC Video Laryngoscope
- Unique Device Identifier (UDI)/Models: 10884521823396/301-000-000 and 10884521776494/301-000-000
Medtronic also provided updated instructions for devices, which can be found by reviewing the FDA notice.
Next steps
For devices that require a full recall, customers are asked to contact Medtronic at (800) 962-9888, Option 2, to initiate a return and replacement. Those with questions are advised to do the same, given the complicated nature of this recall.
For devices that require updated instructions on safe use of the battery, Medtronic said those updates need to be posted on the devices.
In all cases, the company and FDA are asking that the recall notice be shared and reviewed by everyone.

