FDA issues Class I alert on Philips ventilators after 4 injuries
The U.S. Food and Drug Administration (FDA) has issued a Class I recall for multiple lines of Philips ventilators due to the risk of accumulated aerosol deposits, which could damage flow sensors. This may result in a patient experiencing excessive airflow or potentially not receiving the necessary oxygen flow. Additionally, ventilator operators may be unaware of the problem because the accumulation and damage occur gradually.
The issue stems from the use of in-line nebulizer breathing treatments. To use the ventilators safely, operators will need to follow updated use instructions issued by Philips. Ventilators are typically used in hospital or critical care settings, though they may also be used in transport vehicles. Philips manufactures ventilators used for both pediatric and adult patients.
To date, four injuries to patients have been reported as a result of the malfunction. While no deaths have been reported, the recall has been given the FDA’s most serious designation because ventilators are typically used on patients who cannot breathe independently without assistance.
What is being recalled?
Multiple lines of ventilators manufactured by Philips are subject to the recall. Anyone in possession of the devices listed below will need to follow updated use instructions before using any of the systems on patients.
Product Names:
- Trilogy Evo
- Trilogy Evo O2
- Trilogy EV300
- Trilogy Evo Universal
- Aeris EVO
- Garbin Evo
- LifeVent EVO2
Model Numbers:
- DS2110X11B
- DS2100X11B
- DS2200X11B
- DS2000X11B
- VT2110X24B
- LD2110X23B
- SP2100X26B
All serial numbers from all lots of the above products are affected. Any device that has been used with an in-line nebulizer should be considered subject to the recall.
Next Steps
Anyone operating a Philips ventilator should ensure that alarms are set appropriately and are working. Alternate sources for ventilation should be kept on hand in case any of the systems stop working.
Nebulized aerosols accumulate over time and can permanently damage the internal flow sensor. Some systems will need to be repaired. Future use of nebulizers should follow the visual instructions provided in the image below.
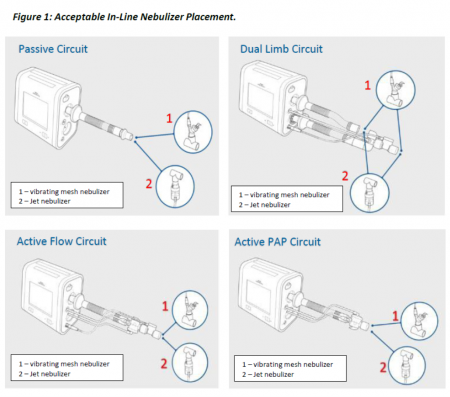
Home care customers are asked to contact Philips at 1-800-345-6443, then select option 4, followed by 5, to have their questions answered by the company.
Hospital customers should call 1-800-722-9377, then select option 2 to speak to a representative.
All known customers were sent a notice in October. However, Philips and the FDA request that the updated instructions be shared. The full notice can be found here.

