FDA approves first-of-its-kind colonoscopy AI
The FDA has granted de novo classification to an AI software module that puts a second pair of eyes on colonoscopy videos in real time and is compatible with any endoscope.
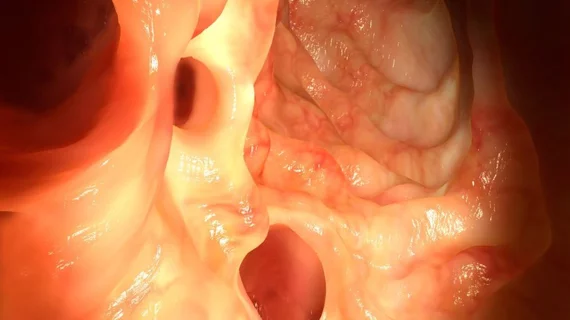
Developed by Cosmo Pharmaceuticals, “GI Genius” flags suspicious lesions by framing them in a green box so the examining gastroenterologist can take a closer look.
The company trained the AI on datasets robust enough that, in a clinical trial, GI specialists using the module caught 13% more biopsy-verified polyps than peers reading the same images without the AI, according to an FDA announcement.
The AI group ordered more biopsies and had slightly more flags of benign lesions but also caught more hard-to-see small and flat polyps.
In a news release sent by Medtronic, the sole distributor of GI Genius in the world, the CEO of the GI Alliance says the technology “can increase the quality of colonoscopies, potentially improving diagnosis and outcomes for colon cancer patients.” This release includes a video demonstration.
A separate release from Cosmo notes that the module is the first of its kind to receive FDA approval via the agency’s de novo pathway.
FDA allows companies to apply for de novo approval, bypassing the slower and more involved 510(k) route, if their product presents low to moderate risk and the market has no substantially equivalent predecessor product.