ACA premiums expected to rise 7% in 2025, report finds
Plans sold on exchanges as part of the Affordable Care Act (ACA) are expected to see a price hike next year.
According to healthcare policy research firm KFF, premiums for plans will rise by around 7% in 2025. However, KFF notes, those who receive subsidies to buy plans are unlikely to experience that full price bump—but ultimately, that means Congress will need to spend more to fund those subsidies.
As for the primary factors driving costs, KFF said insurers cited higher prices from providers and growing demand for expensive GLP-1 drugs, mainly weight loss and diabetes drug Ozempic. But notably, the number of Americans forced to buy insurance on exchanges has also gone up significantly, as Medicaid redetermination pushed them off government plans.
Hospital consolidation, healthcare staffing shortages and general inflation were also cited as contributing factors.
The report from KFF is just a preliminary survey of insurers, and rates will not be finalized until later this year. The expected price change in plans varies dramatically, but most premiums will increase somewhere in the range of 5% to 10%.
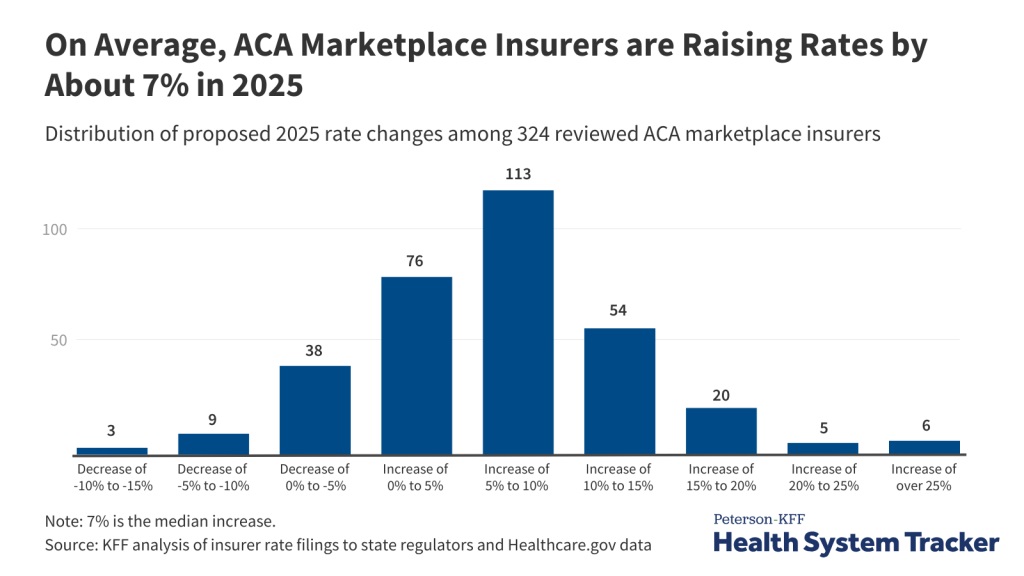
For those who do not receive subsidies to buy insurance from ACA marketplaces, KFF warns, increases may make plans unaffordable, ultimately driving up the count of uninsured Americans.
The full analysis from KFF can be found here.
![Pricing for coronary artery bypass grafting (CABG) varies significantly throughout the United States, according to new findings published in the Journal of the American Heart Association.[1] Researchers emphasized that higher prices were not necessarily associated with better patient outcomes. Photo by Karolina Grabowska via Pexels](/sites/default/files/styles/top_stories/public/2021-08/usa_healthcare_costs.jpg.webp?itok=kJ1yC-u3)
