Students design new face mask that monitors vital signs
A group of students at Cornell University has developed a new face mask design that monitors the wearer’s vital signs.
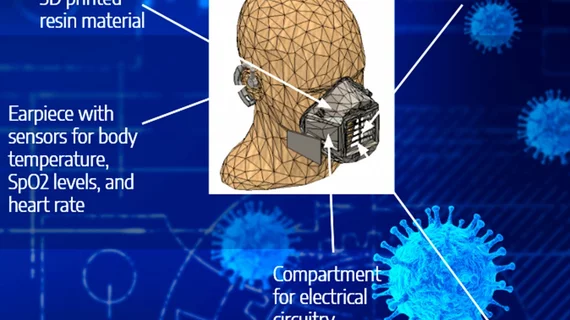
The students submitted their mask, which is made of 3D-printed resin, for Cornell’s AI Health Hackathon in early February. Two dozen teams participated, and this new and improved face mask was declared the winning entry. The team won $2,000 for taking home the top prize and an additional $500 for placing third in a special subcategory focused on assisting with the COVID-19 pandemic.
“If we were going to come up with an idea, this would be the best problem to solve at the moment,” Jason Chen, one of the students involved in the project, said in a Cornell news release.
The team’s VitalMask monitors the wearer’s body temperature, heart rate, blood oxygen levels and respiratory rate. Sensors are placed near the earlobes, nose and mouth.
“These vital signs are transferred in real time to a mobile or desktop app,” Kristin Ong, another student involved in the project, said in the statement. “Not only does the mask help busy medical personnel prioritize patients, it also offers a washable, reusable alternative to standard disposable masks.”
A patent attorney has already helped Chen, Ong and the other participating students file for provisional patents. They also formed a new startup, Vita Innovations, with assistance from a few select mentors.