FDA issues Class I recall of brain fluid drain kits after 15 injuries
The U.S. Food and Drug Administration (FDA) has issued a Class I recall—the most serious type—on several external drainage and monitoring systems (EDMS) manufactured by Medtronic Neurosurgery, branded as Becker and Exacta.
The recall follows 15 patients being injured by the EDMS, which are used to safely drain cerebrospinal fluid from the brain, typically during surgery. The issue arises from leaks and cracks that may be present or form during use, potentially leading to serious health consequences, including loss of cerebrospinal fluid, infections and even death.
To date, no deaths have been reported. In an effort to prevent further incidents, the FDA has issued updated usage instructions to correct the devices. No lines of Medtronic EDMS units are being pulled from the market as part of this recall.
What is being recalled?
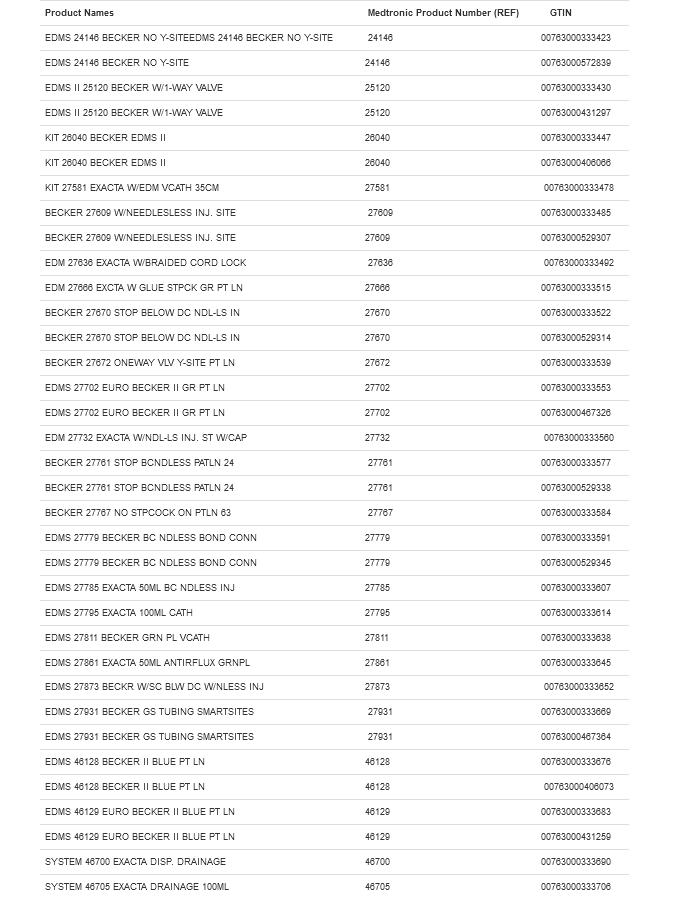
A large number of Becker- and Exacta-branded EDMS kits are included in the recall. Given the scope of this alert, it is recommended to check product names and numbers against the chart below.
The EDMS kits may be available in supply houses or provider settings. In any case, it’s important all customers take notice of the updated use instructions from Medtronic and the FDA.
Next Steps
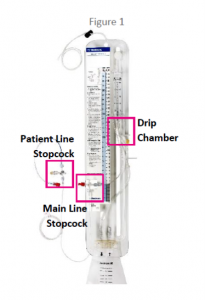
All recalled EDMS devices should be inspected for cracks and leaks. If there are any signs of damage, do not use them. If they have been used recently during a procedure, patients should be carefully monitored for signs of infection.
Medtronic has asked providers to double-check that all stopcocks and connections are free of defects before any of the devices are used on patients. To do this, they recommend using sterile saline before connecting the EDMS to a patient.
Any connection that requires tightening should be secured by hand, not with tools, as over-tightening could cause cracks to form. If a system develops a crack during use, follow sterile procedures to replace it and consider deploying a hemostat or other clamps on the proximal patient line during replacement for increased safety.
All questions or concerns should be directed to Medtronic at 1-800-874-5797, Option 1. Damaged or defective devices can also be returned for a refund.
The FDA is asking all providers to post its notice near the devices. The full recall alert can be found here.

