Eli Lilly releases cheaper, single-use vials of weight loss drug
Pharmaceutical company Eli Lilly announced it will begin selling single-use vials of Zepbound (tirzepatide), a popular weight loss drug, allowing patients to access the medication at a lower price point.
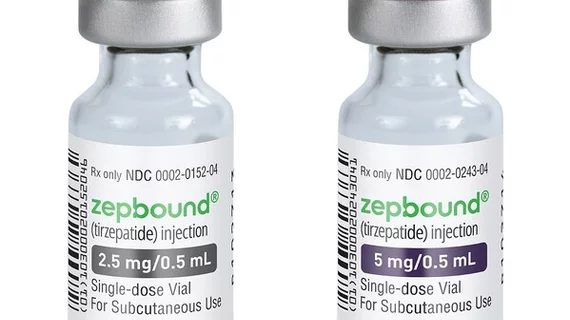
The move will make Zepbound easier to access and increase supply, as it is cheaper than brand-named pens typically used to administer the drug. The single-use vials are easily administered via injection.
However, the company said it will exclusively distribute the new Zepbound through its telehealth platform, LillyDirect, which delivers drugs through the mail. The price point is expected to attract patients paying out of pocket, as many insurance plans will not cover weight loss medications.
"Distributing the vials via this channel ensures patients and providers can trust they are receiving genuine Lilly medicine, building on the company's efforts to help protect the public from the dangers posed by the proliferation of counterfeit, fake, unsafe or untested knock-offs of Lilly's medications," the company said in a statement.
For now, vials cost around $100 for the standard 2.5 mg dose, with 5 mg vials costing $137 a pop. The company said this is less than half the price of similar medications.
Zepbound is approved for use by adults with obesity, or those who are overweight and also have weight-related medical problems, such as diabetes. It’s prescribed for use as part of a larger diet and weight loss program.