9 technology trends and takeaways from RSNA 2022
The expo floor at the Radiological Society of North America (RSNA) is vast and it can be hard to see everything, so here are some interesting new technologies and key trends observed on the floor at the 2022 meeting.
New MRI contrast uses 50% less gadolinium
One of the most practical and helpful new technologies on the RSNA floor was a new magnetic resonance imaging (MRI) contrast agent that uses just half the amount of gadolinium as conventional MRI agents.
The contrast agent gadopiclenol was co-developed by Guerbet and Bracco after major concerns over long-term gadolinium retention from MRI scans in the brain and other tissues were found in studies. There also was a vocal movement by patients claiming they had gadolinium toxicity from MRI contrast agents, and there was a related, notable lawsuit by actor Chuck Norris.
New MRI contrast agent gadopiclenol is sold under the trade name Elucirem by Guerbet and Vueway by Bracco. It was cleared by the U.S. Food and Drug Administration (FDA) in September.
It has a molecular structure with two water molecules rather than one found on all other gadolinium agents. This allows it to bind with tissues in the body for easier and more rapid uptake, so less contrast agent is needed. The molecules are also large, which slows relaxation during MRI scanning.
Guerbet will be producing it domestically in the U.S. in Raleigh, North Carolina, for itself and Bracco. The companies said the U.S. production is also a selling point with hospitals having tightened awareness of international supply chain issues following the recent iodine contrast shortages and several nuclear imaging isotope shortages.
Guerbet said the lower dose of gadolinium will help address concerns about the possible long-term health issues from retained gadolinium. For this reason, Guerbet said it will be targeting the agent for pediatrics and patients who require regular MRI scans.
Photo-counting CT advances at RSNA 2022
Photon-counting computed tomography (CT) detector technology is widely expected to be the way of the future in CT. This is what all the major CT vendors have been saying for several years, as well as experts in radiology and cardiac CT. The technology greatly improves image resolution, improves signal to noise, offers inherent spectral imaging built into all scans, allows for much greater filtering of image artifacts and has the ability to see through calcium and metal implants.
The first photon system from Siemens was cleared by the FDA in 2021. This year at RSNA, a second FDA-cleared, photon-counting CT system was being shown by Samsung, the OmniTom Elite Photon-Counting Detector (PCD). It is a compact head scanner for head trauma and brain imaging.
GE Healthcare also showed its work-in-progress deep silicon photon-counting CT detector technology at RSNA. It also announced a new chapter in its development with the first prototype scanner now operating at the University of Wisconsin in Madison. UW scanned its first two patients with the system the week of Thanksgiving a few days prior to RSNA.
Photon-counting CT technology and small studies using it were discussed in several sessions at RSNA 2022. It was one of just three new technologies highlighted as a key takeaway by Jorge Soto, MD, chair of the RSNA Annual Meeting Program Planning Committee, and chief of radiology, Boston Medical Center, in an overview at the meeting.
Hear more of Soto's overview in the VIDEO: Key takeaways from RSNA 2022.
FDA-cleared, cold-cathode mobile DR X-ray system at RSNA 2022. The Micro-X Rover uses carbon nanotubes rather than the standard filaments used in X-ray tubes for the past century. The nano tubes produce an electron beam in the tube without producing heat, which is the majority of the energy released in standard tubes. This eliminates the need for cooling systems, the related structural metal supports and motorized drive system because of a massive weight reduction.
Cold-cathode X-ray and CT technology
Cold cathode X-ray tubes have the possibility of revolutionizing medical imaging and reducing costs and weight of current X-ray based imaging systems. There have been a lot of engineering issues that needed to be overcome, but vendor Micro-X at RSNA 2022 said they have solved the issue of tubes wearing out, and they displayed an FDA-cleared mobile digital X-ray system on the floor. The company also showed a miniature head CT scanner using the same carbon nanotube cold cathode X-ray technology, which is already being deployed in Australia.
The Rover mobile DR system uses carbon nanotubes to create a beam of electrons in the tube that hit a metal target to create X-rays. Conventional tubes used over the last century use a light-bulb like filament that produces more heat than electron beam or X-rays, so systems need passive and active cooling systems. The weight of these systems and conventional tubes requires heavy structural support and motorized systems because of the weight. But these cold cathode systems are only a faction of the weight because they are made with minimal metal and carbon lightweight materials. The Rover is 220 pounds (100 kg), so it can easily be pushed without a motorized drive system.
The company has a 10 year warranty on the tubes, which operate in the 20-30 mA range, but the vendor said they have been running them up to 90 mA. The company also partnered with Varex for its digital X-ray detectors used on the Rover.
Micro-X was the OEM maker of the Carestream Nano carbon nanotube system that made headlines a few years ago at RSNA as the first commercial, FDA-cleared cold-cathode X-ray system. The Rover is the Micro-X version of the Nano system.
The vendor said it is also developing a new, lightweight, cold-cathode X-ray screening system for the U.S. Department of Homeland Security.
Another vendor, Nanox, has a lightweight, cold-cathode multi-source X-ray system pending regulatory review in both Europe and with the FDA.
Read more on the Nanox business model based around its cold-cathode technology in the article Can cold-cathode X-ray combined with teleradiology and AI eliminate health disparities?
FDA cleared radiology artificial intelligence algorithms
Conversations on artificial intelligence (AI) at RSNA have moved way beyond 101-level discussions on how AI apps work from just a few years ago. Today, with several hundred AI algorithms now cleared by the FDA, the conversations have changed to acceptance of AI and the questions are now about how AI will help the radiologist address problems with workflow or improve imaging.
Seamless integration of AI Information imaging systems or radiology IT systems is also a key trend. Vendors, both PACS and AI, realized no one will used the technology if it requires a separate workflow or login. The AI needs to be fully integrated into the workflow, or else most radiologists have no interest.
This has led to PACS, enterprise imaging and advanced visualization vendors across the RSNA expo floor to highlight how integrated they are with AI partners. Numerous vendors offer their own versions of AI app stores where their hospital customers can pick which AI apps they want to be added to their tab and they will be fully integrated into their workflows.
Radiology IT vendors have focused on partnerships with emerging AI vendors, while just a few years ago many large radiology vendors wanted to try and develop their own AI applications. Over the past couple of years this approach has been abandoned in favor of AI partnerships. Often, these key partner AI vendors now work with numerous large PACS or imaging systems vendors to supply their algorithms for integration. This is similar to third-party vendors that supply advanced visualization software, like TomTec, ContextVision and TeraRecon.
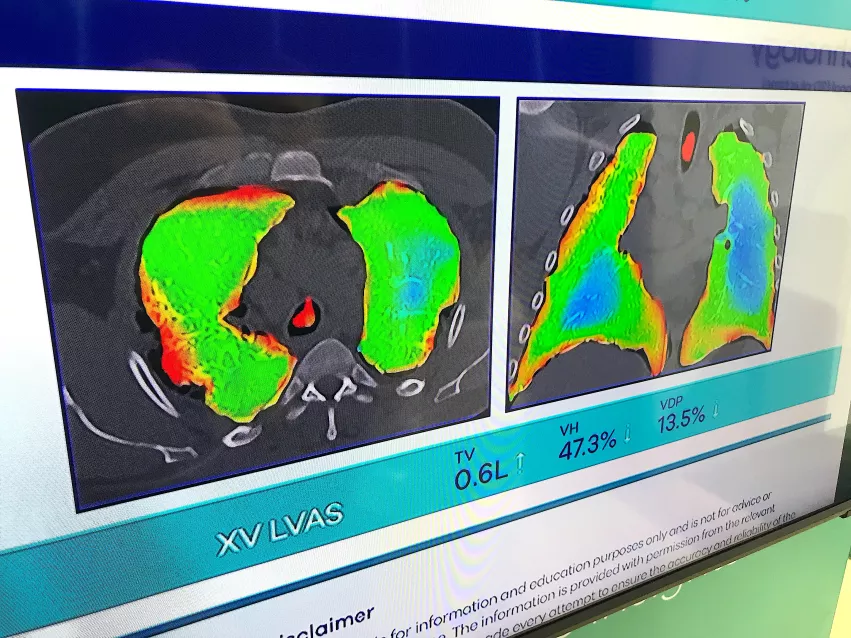
Dynamic X-ray lung and airflow assessment without contrast
One of the most interesting new technologies on the floor was an X-ray lung airflow imaging system that does not use any sort of contrast. Displayed by the startup 4D Medical, the software uses multiple views from a fluoroscopy X-ray system, or the vendor's dedicated lung XV Scanner. The dedicated system uses four pulsed X-ray beams from different angles as the patient sits in a chair with the detectors behind them. The software calculates the airflow from the movement of the lung tissue.
The company said it is also developing this technology further to show blood perfusion in the lungs.
Glassless digital X-ray plates help prevent damage when dropped
One of the biggest obstacles that prevented the rapid transition from computed radiography cassettes to digital radiography (DR) X-ray a decade ago was the high cost of the plates and the equally high replacement cost when they get dropped and broken. A glass substrate is used to attach the detector elements, but the glass can crack or shatter when dropped by accident, which is a common in busy imaging departments or with mobile DR systems. Many vendors began offering guarantees or reduced cost replacement programs to try and entice customers to switch to DR, which has since become the dominant X-ray technology today.
But the durability of DR plates got a boost from a couple of vendors that now offer glassless plates. Fujifilm introduced a glassless plate in 2019. This year, Konica-Minolta introduced its first glassless Aero DR plate.
Glass is traditionally used in DR detector plates as a substrate for the detector elements, but replacing it with a polymer construction makes it more durable, both vendors said. The drop height rating for the plate from Konica plate is 1.5 meters. They said the electronics in the plate can still be broken when dropped, but compared to the traditionally much more fragile plate itself, it now has a much better survival rating.
Konica also used the innovation of a capacitor rather than a traditional battery to power the wireless plate for up to 3 hours. The vendor also added deeper grooves on the sides of the antibacterial coated cardio fiber plate housing to make it easier to grasp with one hand. Eliminating the glass also significantly reduced the weight of the plate, making it less likely to be dropped.
Seno Medical showed the new breast imaging modality of opto-acoustic ultrasound (OA/US). It uses six separate images to show the functional and anatomic features of breast tissue and immediately displays features that differentiate tumor neo-angiogenesis. This may prevent the need for additional testing or biopsy. The system just received U.S. FDA PMA clearance.
Two new breast imaging modalities aid women with dense breasts
There were two innovative new breast imaging technologies on the floor, both using ultrasound and elastography to better understand if a suspicious area is cancer or not without the need to biopsy every patient. Both technologies are also designed to help image women with dense breasts to be able to see through the dense tissue that masks cancers on traditional mammograms.
Seno Medical showed the new breast imaging modality of opto-acoustic ultrasound (OA/US). It uses six separate images to show the functional and anatomic features of breast tissue and immediately display features that differentiate tumor neo-angiogenesis. This may prevent the need for additional testing or biopsy. The system just received U.S. FDA PMA clearance and the vendor celebrated with champagne in the booth.
Read more about this new technology.
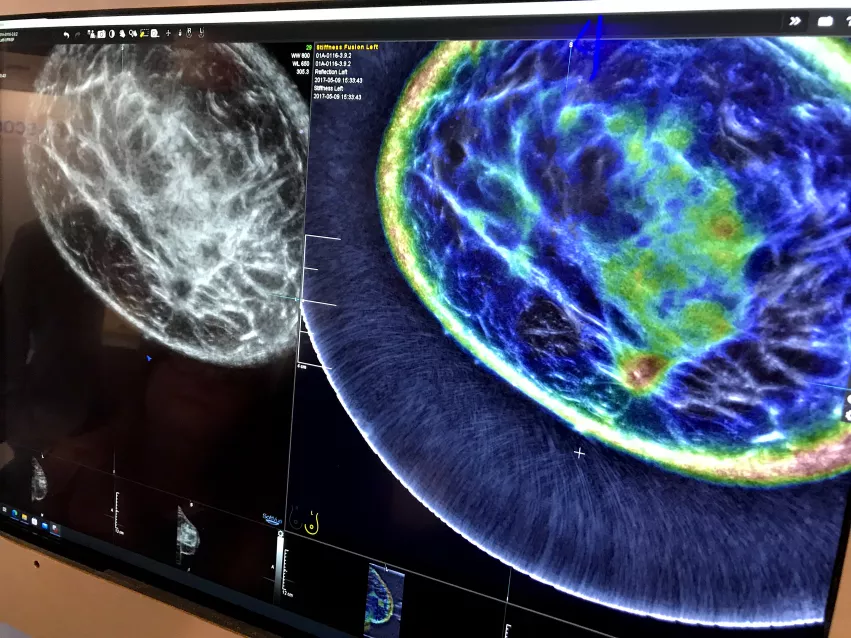
The second vendor Delphinus offers whole breast 3D ultrasound tomography. To use the system the patient lays in a prone position on a table with their breast inserted into a hole in the table. It is filled with water and a light suction device pulls the breast down to elongate without compression.
An example of the Delphinus whole breast ultrasound tomography technology. A ring transducer surrounding the breast creates an image of the whole breast, similar to MRI. It also uses reflection, sound speed and attenuation to characterize the tissue structure, as seen in the color-coded image, to help identify cancers.
The color coded image shows areas of breast density and attenuation that can be compared to the 2D ultrasound image. It resembles and reads much like a breast MRI exam. A ring-shaped ultrasound transducer surrounds the breast and gathers not only imaging data, but also information on tissue attenuation to evaluate stiffness. It is said to be much more comfortable for patients.
Due to the ring transducer, the ultrasound image looks like a cross between a CT and an automated breast ultrasound exam. The color coded overlay on the image is read similar to an MRI.
Studies are being conducted now to see if the technology can show early changes in the tissue due to chemotherapy treatment, even before the tumor begins to shrink.
Syringeless contrast media injectors like the GE Ulrich CT Motion gained a lot of interest in 2022 because of the iodinated contrast shortage. These systems use rollers to squeeze precise amounts of contrast from a large bolus container for multiple patients, helping conserve a significant amount of contrast.
Conserving iodine contrast becomes a priority after 2022 supply chain shortage
With the iodine contrast shortage earlier in 2022 caused by strict COVID shutdowns in China, health systems have become more aware of supply chain issues and are looking for ways to conserve contrast. GE Healthcare was at the center of the iodine shortage this year because it is the largest producer of iodinated contrast with its factory in Shanghi, China. At RSNA, the vendor expanded the section of its booth involving contrast to highlight its efforts to address possible future shortages and recycling of unused contrast.
GE said there has been a large amount of interest in its syringe-less Ulrich CT Motion contrast injector. It uses a large bolus of contrast and a roller system to push a precise amount on contrast down the IV tubing. This can save contrast between patients by eliminating waste that is common in the smaller, single-use vials. The injector helped reduce contrast usage in a study at the University of Washington, saving about 30 ml of contrast per patient. This allowed 6-7 patients to be scanned with a 500 ml bolus, rather than the usual 3-4 patients using a traditional syringe injector system.
GE also explained that it is increasing its production capabilities at its contrast factory in Norway so it will not be caught in an other shortage. GE said they have received new interest in their contrast recycling program it started years ago, but became more important during the shortage. The program collects used vials and bolus containers of contrast so the small amounts left inside can be recycled for reuse.
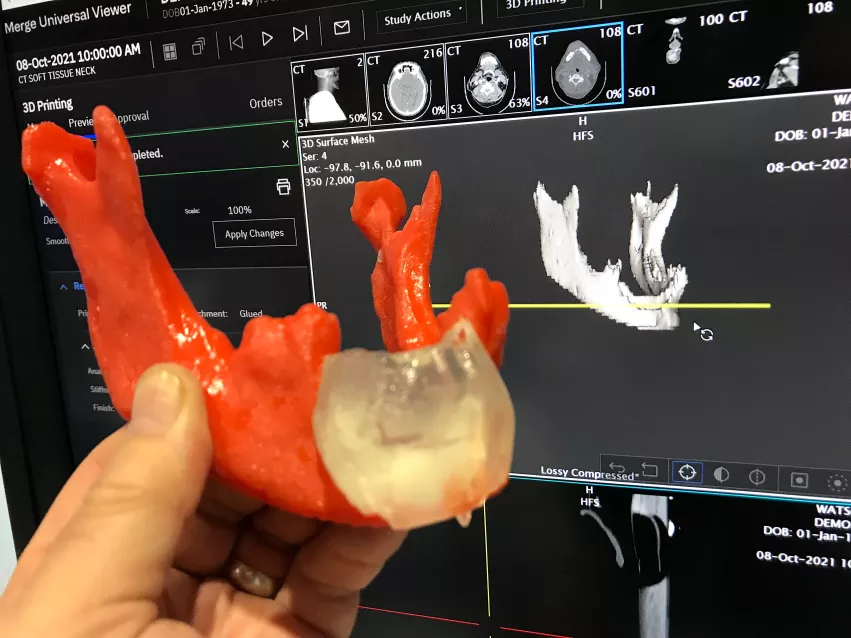
Expanding access to 3D printed medical models
Many hospitals do not have 3D print labs and only about 10% of U.S. surgeons have access to the technology to help with procedural planning. The printer company Ricoh, in partnership with Merge/Merative, now offers a 3D contract printing service. Users of the Merge PACS system have an app that allows clinicians to take 3D DICOM CT datasets and convert them into STL files for printing. The radiologist can then place an order online and receive the 3D printed medical grade project in back within a week.
The goal of the business model is to simplify the 3D printing file creation and ordering of medical grade prints. 3D medical printing has grown significantly in the past few years to help with planning complex surgeries, orthopedic implants and interventional and structural heart procedures. RSNA has had a 3D printing section of vendors on the expo floor now for several years.