VA first to cover new ALZ drug
A new Alzheimer’s disease drug, Leqembi (lecanemab), will be covered by the Veterans Affairs for some patients, making the VA the first insurer to cover the drug.
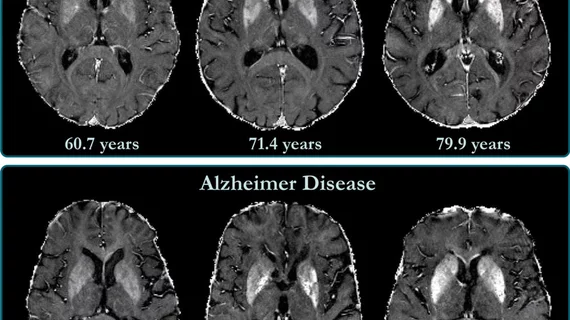
Leqembi was recently approved by the U.S. Food and Drug Administration (FDA) through the agency's Accelerated Approval pathway at the start of 2023. The drug has given many high hopes for improved treatment of Alzheimer’s disease and dementia, which impacts an estimated 6.5 million Americans aged 65 and older.
The drug was developed by Japanese pharmaceutical company Eisai and is based on the observed reduction of amyloid beta plaque, a marker of Alzheimer’s disease. The Centers for Medicare and Medicaid Services (CMS) recently declared it would not change its drug restrictions for Alzheimer’s drugs, after the agency was reconsidering the national coverage determination (NCD) for Food and Drug Administration (FDA)-approved monoclonal antibodies.
“The VHA’s careful consideration and timely action to make Leqembi available approximately two months after the FDA approved Leqembi under the accelerated approval pathway shows its continued commitment to veterans living with [Alzheimer’s disease],” Eisai said in a statement March 14.
The move shut down a petition for wider coverage of Leqembi, which has been found to reduce amyloid plaque buildup in the brain, a marker of Alzheimer’s disease. CMS is unlikely to grant wider coverage of the drug until the FDA grants traditional approval for Leqembi, which could come in July.
“If approved under the traditional pathway, the FDA will update the label for Leqembi, which will include new data that has been evaluated by the FDA,”Eisai said. “Eisai looks forward to sharing additional high-quality data as it becomes available and to continuing discussions with the VHA as the company prepares for the FDA’s potential conversion of Leqembi’s accelerated approval to a traditional approval.”
The VA’s decision to cover Leqembi in certain circumstances is good news for those hoping to see wider adoption of it. Leqembi is the first drug that has shown to slow Alheimer’s disease. The VA published its list of requirements for patients requesting it. According to the VA, more than 168,000 VA patients are estimated to have Alzheimer’s dementia.