Global Pharma Healthcare recalls eye drops over possible contamination
Global Pharma Healthcare has voluntarily recalled all lots within expiry of their Artificial Tears Lubricant Eye Drops due to possible contamination, the U.S. Food and Drug Administration (FDA) announced.
The drops were linked to 55 reports of adverse events, including eye infections, permanent loss of vision and one death with a bloodstream infection. The FDA became aware of the possible contamination from the Centers for Disease Control and Prevention (CDC), which alerted the agency to an investigation of a multi-state cluster infections. According to the CDC, the EzriCare brand drops lead to infections in 12 states between May 17, 2022, to January 19, 2023.
“Review of common exposures among patients identified that the majority of patients used artificial tears prior to identification of VIM‐GES‐CRPA infection or colonization,” the CDC said in a press release. “The most common brand reported was EzriCare Artificial Tears, a preservative‐free product dispensed in multidose bottles.”
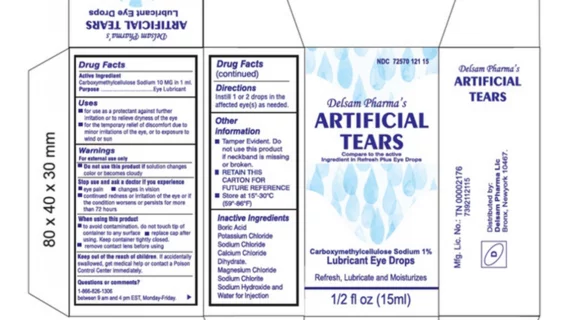
The eyedrops, Artificial Tears (carboxymethylcellulose sodium) Lubricant Eye Drops, 10 mg in 1 mL, ½ fl oz (15 ml) bottle, are used to temporarily relieve irritation or to dryness for instances of minor eye irritation or exposure to wind or sun. The product is packaged in a bottle with a safety seal and are placed in a carton box Ezricare NDC 79503-0101-15, UPC 3 79503 10115 7; Delsam Pharma’s NDC 72570-121-15, UPC -72570-0121-15.
Lab testing of EzriCare Artificial Tears by CDC identified the presence of VIM‐CRPA in opened EzriCare bottles. The CDC is testing if the VIM-CRPA identified in the bottles matches the outbreak strain. Additionally, the agency is testing unopened bottles of the brand’s eye drops.
“CDC recommends that clinicians and patients immediately discontinue the use of EzriCare Artificial Tears until the epidemiological investigation and laboratory analyses are complete,” the CDC said.
Global Pharma is notifying its distributors, Aru Pharma Inc. and Delsam Pharma, of the possible contamination. The company requests that wholesalers, retailers and customers who have the recalled product should stop use.
Consumers with the recalled products can contact the distributors, Aru Pharma/Ezricare, while adverse reactions or quality problems experienced with use of the product should be reported to the FDA's MedWatch Adverse Event Reporting program online, by mail or by fax.