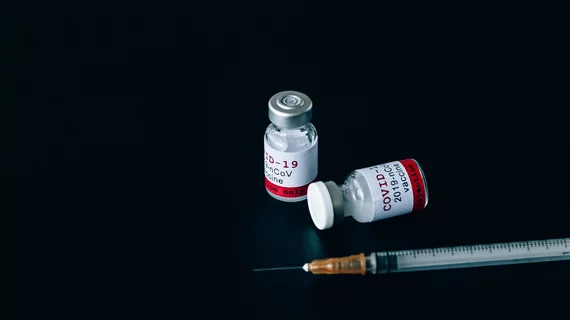
COVID booster shots will become available in September
COVID-19 booster shots from Pfizer-BioNTech and Moderna will be available starting the week of September 20, the White House announced.
The booster shots will be administered by the Department of Health and Human Services, pending FDA approval. The shots are intended to be administered eight months after an individual receives a second dose of the vaccine.
“The COVID-19 vaccines that are authorized in the United States have been remarkably effective, even against the widespread Delta variant,” Surgeon General Vivek Murphy said in a statement. “But we know that even highly effective vaccines become less effective over time.”
When the booster shots become available, many frontline workers, including healthcare professionals, teachers and seniors, will be eligible. However, the plan is still waiting on FDA approval.
“I want to be very clear: This plan is pending the FDA conducting an independent evaluation of the safety and effectiveness of a third dose of the Pfizer and Moderna mRNA vaccines and the CDC’s Advisory Committee on Immunization Practices issuing booster dose recommendations based on a thorough review of the evidence,” Murphy said.
The new plan comes as COVID-19 cases continue to rise. More than 621,000 Americans have lost their lives due to the virus, according to the CDC. In addition, more than 37 million cases were reported in the last 30 days. More than 72% of U.S. adults are fully vaccinated.