Hackensack Meridian teams up with AI specialists for brain tumor care
Hackensack Meridian Health, a not-for-profit healthcare organization and the largest healthcare network in New Jersey, has teamed up with AI company Neosoma to treat complex brain tumors.
Neosoma, based in Massachusetts, uses AI to help clinicians advance the treatment of brain cancers. The partnership includes clinical data sharing, clinical research and strategic investment from Hackensack Meridian to support Neosoma’s method of imaging, tracking and collecting data on numerous types of brain tumors, including glioblastomas.
Hackensack Meridian physicians will use Neosoma’s software in clinical practice, starting with Hackensack Meridian JFK University Medical Center. Physicians will give input and feedback for development of future software including neurosurgeons, neuro-oncologists, neuroradiologists, radiation oncologists and other clinicians. The data-sharing aspect includes product R&D efforts such as collaborating with Anthology Diagnostics for genomics data and capabilities, among other benefits.
"We are committed to investing in research and innovative therapies to live our mission to transform healthcare and give our patients the best possible outcomes,'' Robert C. Garrett, CEO of Hackensack Meridian Health, said in a statement. "Partnering with Neosoma Inc. is a great way to continue to develop potential game changing therapies to treat these challenging cancers."
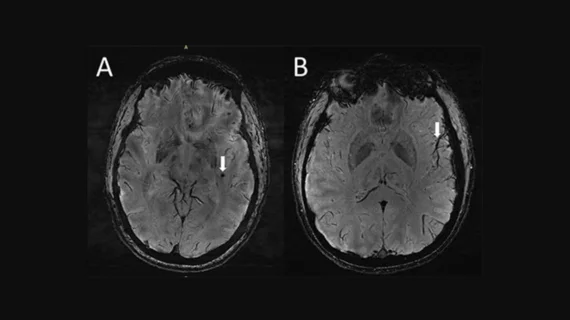
Neosoma received clearance from the U.S. Food and Drug Administration (FDA) for its first AI-based neuro-oncology software device, Neosoma HGG (High-Grade Glioma). Within clinical practice, physicians use brain MRIs to evaluate the details and changes in a brain tumor. The AI technology can help physicians better plan procedures, assess post-procedural results, guide chemo and immunotherapy treatments, track patients longitudinally, support an improved patient experience and help lead to improved treatment, according to the company.
“The technology produces precise and accurate brain tumor analysis on MRIs, providing physicians with critical insights to guide treatment decisions,” the company said in a press release.
The partnership comes as AI continues to be adopted in the healthcare space. The FDA has cleared more than 500 AI algorithms over the past several years, mostly focused on the imaging space.