Fujifilm CMO on the company’s growing healthcare portfolio and the trends of tomorrow
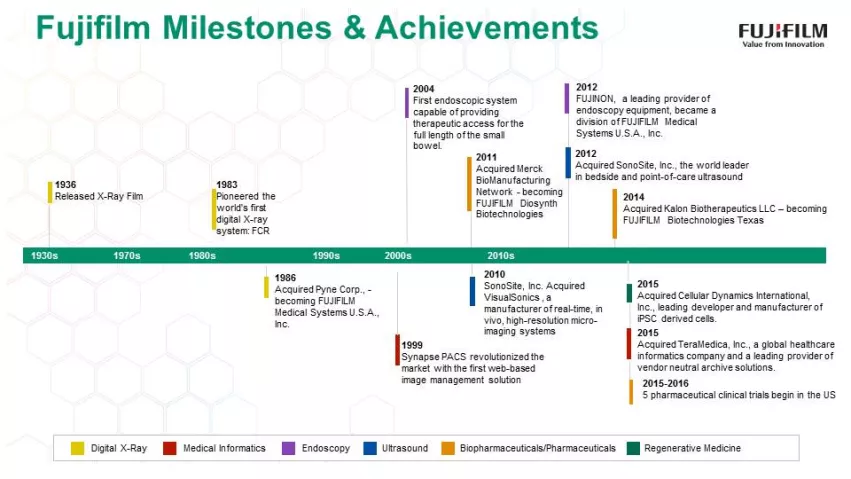
Some people may still primarily associate Fujifilm with its film and camera business, but that’s just part of the picture. Fujifilm’s ties to healthcare date all the way back to 1936, when it released its very first x-ray film. Today, Fujifilm is a large global company—it reported revenues of more than $22 billion in the last year—and its healthcare business alone was responsible for approximately $3.8 billion in revenue.
The Fujifilm healthcare portfolio includes endoscopy, ultrasound, medical informatics, digital x-ray systems, women's healthcare, and much more. And as Diku Mandavia, MD, chief medical officer and senior vice president of both FUJIFILM Medical Systems U.S.A., Inc. and FUJIFILM SonoSite, Inc. explains, the company’s commitment to healthcare is stronger now than ever before.
“We pioneered the world’s first digital X-ray system,” says Mandavia. “Ever since, Fujifilm has remained a leader, offering products and services that maximize workflow efficiency and provide exceptional image quality with renowned reliability to meet our customer needs.”
To appreciate Fujifilm’s healthcare business in the United States, it’s easiest to categorize the company’s solutions into categories: diagnostic imaging, medical informatics, biopharmaceuticals, pharmaceuticals and regenerative medicine.
From an unrivaled selection of digital x-ray systems, to the Synapse IT brand of VNA (vendor neutral archive), PACS, and RIS to SonoSite ultrasound, Fujifilm leverages the products, services, and expertise to enhance the quality of care for patients. Fujifilm has successfully transformed its business structure for growth by expanding from traditional photographic film to other priority business fields. Positioning the healthcare business as one of its key growth areas, Fujifilm is seeking to address prevention, diagnosis, and treatment comprehensively.
An adventurous path
Mandavia traveled an adventurous path on his way to where he is now with Fujifilm. In 1991, he was accepted by a prestigious emergency training program in Los Angeles and found himself treating victims of stabbings, gunshot wounds, and other forms of trauma on a daily basis. In this role, Mandavia witnessed modern ultrasound in action, and the power and promise of that technology changed his career forever.
“It was a lightbulb moment for me,” Mandavia says. “This technology was going to have a big impact on patient care. Previously, many of these patients died because we didn’t know if they had internal bleeding or not, or they went to get a CT scan, which at the time, took a long time to obtain and delayed treatment. Bedside ultrasound was a game changer.”
Mandavia became an expert in clinical ultrasound and eventually joined SonoSite - the world leader in point of care ultrasound, which was acquired by Fujifilm in 2012. Mandavia says his career path—starting as an emergency physician and becoming an executive within a global healthcare company—gives him a unique perspective on the ever-evolving world of modern medicine.
“That’s my contribution to Fujifilm: being able to connect the provider and patient side to product development and the commercialization required to get a solution into a doctor’s hands,” Mandavia says. “Being a physician within the executive leadership team, I look at things with a different lens than someone else might within the company.”
Looking at modern healthcare as a whole, Mandavia notes that ultrasound, the technology that first grabbed his imagination more than two decades ago, is currently experiencing a full renaissance. Due to both prevalent patient concerns over the radiation dose associated with CT scans and new advancements in ultrasound technology, he says, ultrasound has shifted from being a largely forgotten modality to playing a ubiquitous role in hospitals and clinics all over the world.
“You see not just one specialty using ultrasound; you see all specialties starting to use it,” Mandavia says. “This is a trend that is happening, and you’re going to see more of it. Many medical schools today are already training students as early as the first year of medical school to use ultrasound. My prediction is, all physicians will utilize highly mobile ultrasound in the future.”
Looking ahead
What’s next for Fujifilm’s healthcare business? Mandavia says the company is always looking forward, and investments over the past decade in pharmaceuticals, biopharmaceuticals and regenerative medicine have created exciting new advancements. Fujifilm has accepted the research and development investment required for success in the pharmaceutical industry.
Fujifilm is creating novel drugs with unique mechanisms of action to meet unmet medical needs focusing on cancer, infectious diseases and central nervous system diseases. Fujifilm currently has three Phase I clinical trials at the University of Texas MD Anderson Cancer Center, one of the world's most distinguished facilities for cancer research and treatment. Furthermore, Fujifilm is working with the governments of France, Guinea and Japan to treat the recent outbreak of Ebola in West Africa.
“Fujifilm has endured as a leader and has leveraged its fundamental and core technology to become a global presence known for innovation in healthcare,” Mandavia says. “Building on our pioneering diagnostic imaging technologies, we are expanding into new areas and offering a comprehensive healthcare portfolio.”