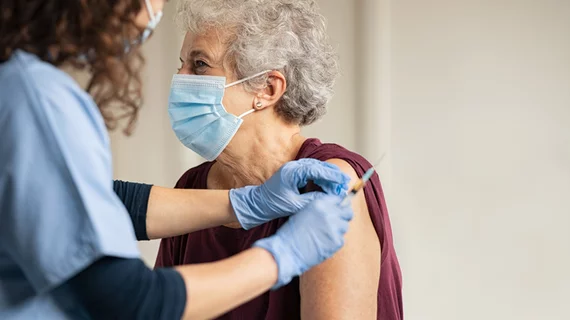
Half of US adults have received one COVID-19 vaccine dose
Half of U.S. adults have received at least one dose of a Covid-19 vaccine, the Centers for Disease Control and Prevention disclosed. About one-third of adults are also fully vaccinated.
The latest figures reveal just how robust the U.S. national Covid-19 vaccination program is, with three FDA-approved vaccines. Roughly 211 million vaccine doses have been administered in the U.S. as of April 19, according to the CDC. However, the CDC recently recommended a pause of the Johnson & Johnson Covid-19 vaccine after six women experienced a rare blood clot associated with the vaccine.
The latest figures from the CDC comes as the number of Covid-19 cases and deaths continue to rise across the nation. At the time of publication, there were nearly 31.5 million Covid-19 cases and 564,292 reported deaths.
Among seniors, who have been hardest hit by the disease, 80.1% have received one vaccine dose, and nearly 65% were fully vaccinated.
Despite the pause on the Johnson & Johnson vaccine, which is administered in one dose, U.S. public health officials remain bullish on the vaccination rollout. The current pace of vaccinations tops 3 million per day, putting President Biden on track to meet his goal of 200 million doses within his first 100 days in office, according to a statement from Jeff Zients, White House Covid-19 response coordinator.
“Based on actions taken by the President earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans,” Zients said.