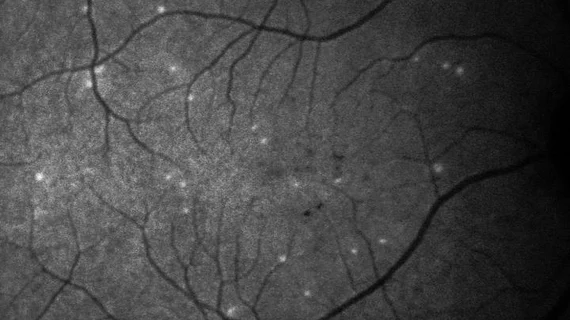
AI-powered test spots early signs of glaucoma progression
Researchers have developed a new AI-powered test capable of assessing glaucoma progression 18 months earlier than other methods, sharing their findings in Expert Review of Molecular Diagnostics. The test, named DARC (Detection of Apoptosing Retinal Cells), may also have a second important use—improving the treatment of COVID-19 patients.
Physicians perform DARC by injecting a fluorescent dye into the patient’s blood. That dye latches on to retinal cells, illuminating any in the process of apoptosis. A convolutional neural network was then introduced to provide consistency to the assessment of DARC results—and the team achieved considerable success.
Overall, the algorithm helped evaluate DARC results of 60 patients—one in three had glaucoma, the others were healthy controls. Each patient’s health was followed up on 18 months later, helping the researchers see how their health has progressed and if the test had been correct.
For spot detection, the algorithm had an accuracy of 97%, sensitivity of 91.1% and specificity of 97.1%.
“We have developed a quick, automated and highly sensitive way to identify which people with glaucoma are at risk of rapid progression to blindness,” lead researcher Francesca Cordeiro, MD, PhD, University College London Institute of Ophthalmology, said in a statement.
“Being able to diagnose glaucoma at an earlier stage, and predict its course of progression, could help people to maintain their sight, as treatment is most successful if provided at an early stage of the disease,” added first author Eduardo Normando, MD, PhD, of Imperial College London. “After further research in longitudinal studies, we hope that our test could have widespread clinical applications for glaucoma and other conditions.”
One condition the team is currently exploring is lung disease related to the new coronavirus. By the end of this year, the study’s authors hope their algorithm can play a key role caring for patients impacted by COVID-19.